

Also, the methodological approaches currently used and reported underutilise the informational contents of EEG signals. Unfortunately, to date there is no established methodological approach to the design of QEEG derived biomarkers, in particular, in an automatic, objective and reproducible way. QEEG has been shown to distinguish between attention deficit/hyperactivity disorder (ADHD) responders and non-responders to stimulant medication 7, 8, 9. The identification of treatment responsive QEEG subtypes has been described in depression 2, 3, obsessive compulsive disorder 4, 5 and schizophrenia 6, suggesting that understanding the underlying neurophysiology of the patient can contribute significantly to treatment optimization.

Indeed, recent evidence indicates that quantitative electroencephalogram (QEEG) is a powerful tool in pharmaco-EEG applications. Specifically, electroencephalography (EEG)-based markers have the potential of serving as such a battery, due their noninvasiveness and well-established recognition in clinical practice.
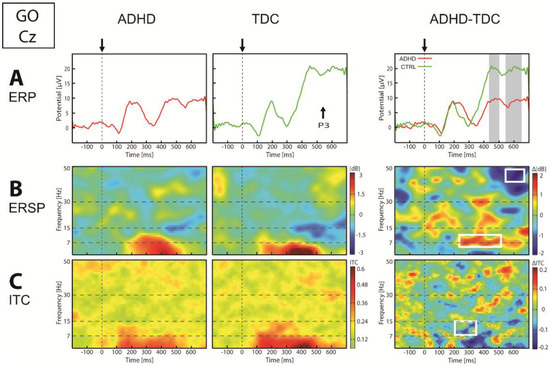
The existence of a battery of non-invasive biomarkers capable of identifying neurogenic alteration of normal functioning and capturing the response to pharmacological agents would be of great importance in clinical practice. This applies specifically to diseases of pathoneurological origin, where the cognitive and behavioural health of the individual is affected. In particular, non-invasive biomarkers are of high value in diagnosing diseases and evaluating disease progression and the efficacy of medication. Biomarker discovery is essential to drug development, and also constitutes a formidable challenge in evaluating the effects of newly developed pharmacological agents.

Neurogenic cognitive and behavioural disorders constitute an ever-growing challenge to societies 1, barely met by the continuing development of pharmacological treatments. The methodology proposed is of generic use as an approach to investigating thoroughly the dynamics of the EEG spectral power. Composite biomarker evaluation confirms their validity for genetic model stratification and the effects of the pharmacological agents used. Results of the analysis of variance (ANOVA) and t-test show benefits in pharmacodynamic parameters, especially the slope parameter. We apply Fisher discriminant analysis (FDA) to identify dominant discriminants to be heuristically consolidated into several new composite biomarkers. To assess individual EEG patterns quantitatively, we use an integrated methodological approach, which consists of calculating the mean, slope and intercept parameters of temporal records of EEG spectral power using a smoothing filter, outlier truncation, and linear regression. We inject the two agents into the spontaneously hypertensive rat (SHR) model of ADHD, the Wistar-Kyoto rat (WKY), and the Wistar rat (WIS), and record their EEG patterns. We apply this methodology to investigate the pharmacodynamic effects of methylphenidate (MPH) and atomoxetine (ATX) on attention deficit/hyperactivity disorder (ADHD), using rodent models. We propose a novel semi-automatic approach to design biomarkers for capturing pharmacodynamic effects induced by pharmacological agents on the spectral power of electroencephalography (EEG) recordings.


 0 kommentar(er)
0 kommentar(er)
